A moonshot in autoimmune disease. Elevating cell therapy.
Innovating Treg engineering and manufacturing to turn the tide in serious autoimmune and inflammatory disease
Our Approach
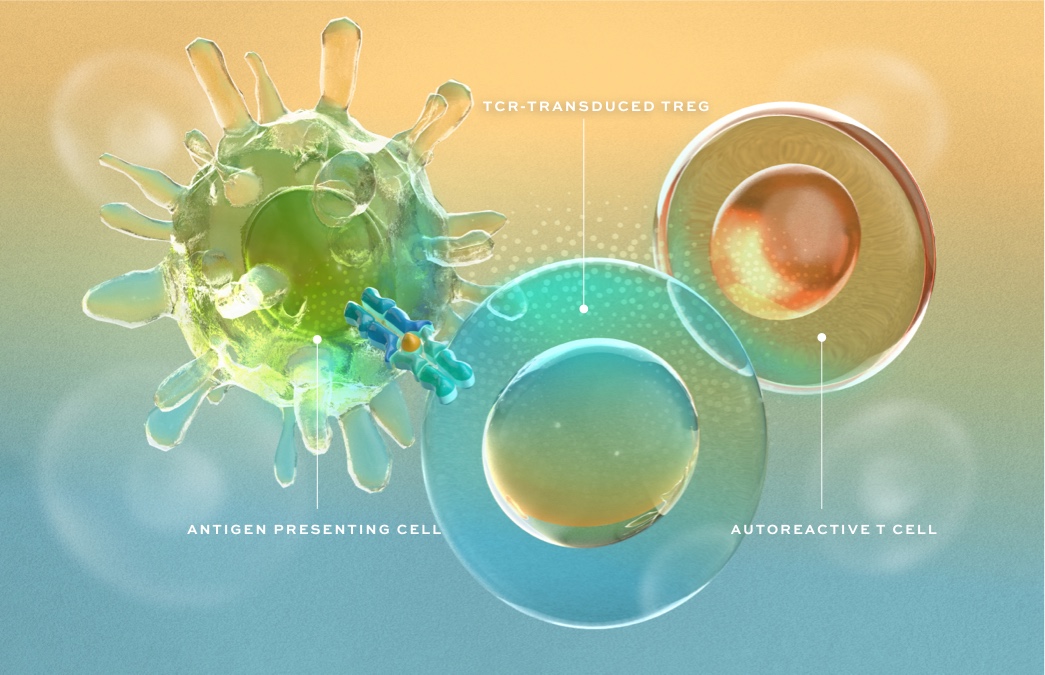
We are bringing an entirely new approach to the treatment of autoimmune disease by engineering regulatory T cells (Tregs) as targeted therapies designed to stop immune-mediated destruction, restore homeostasis – a state of harmony – and promote repair in affected tissues.

Product Engine
Based on the innovative work of our founders, we are developing a differentiated, cutting-edge product engine to create engineered Treg cell therapies for autoimmune and inflammatory disease.
MHC Class II-Restricted TCR Discovery
Expertise and technologies to identify and characterize the right TCR in each disease for tissue-targeted Treg activation and to establish residency in affected tissues.
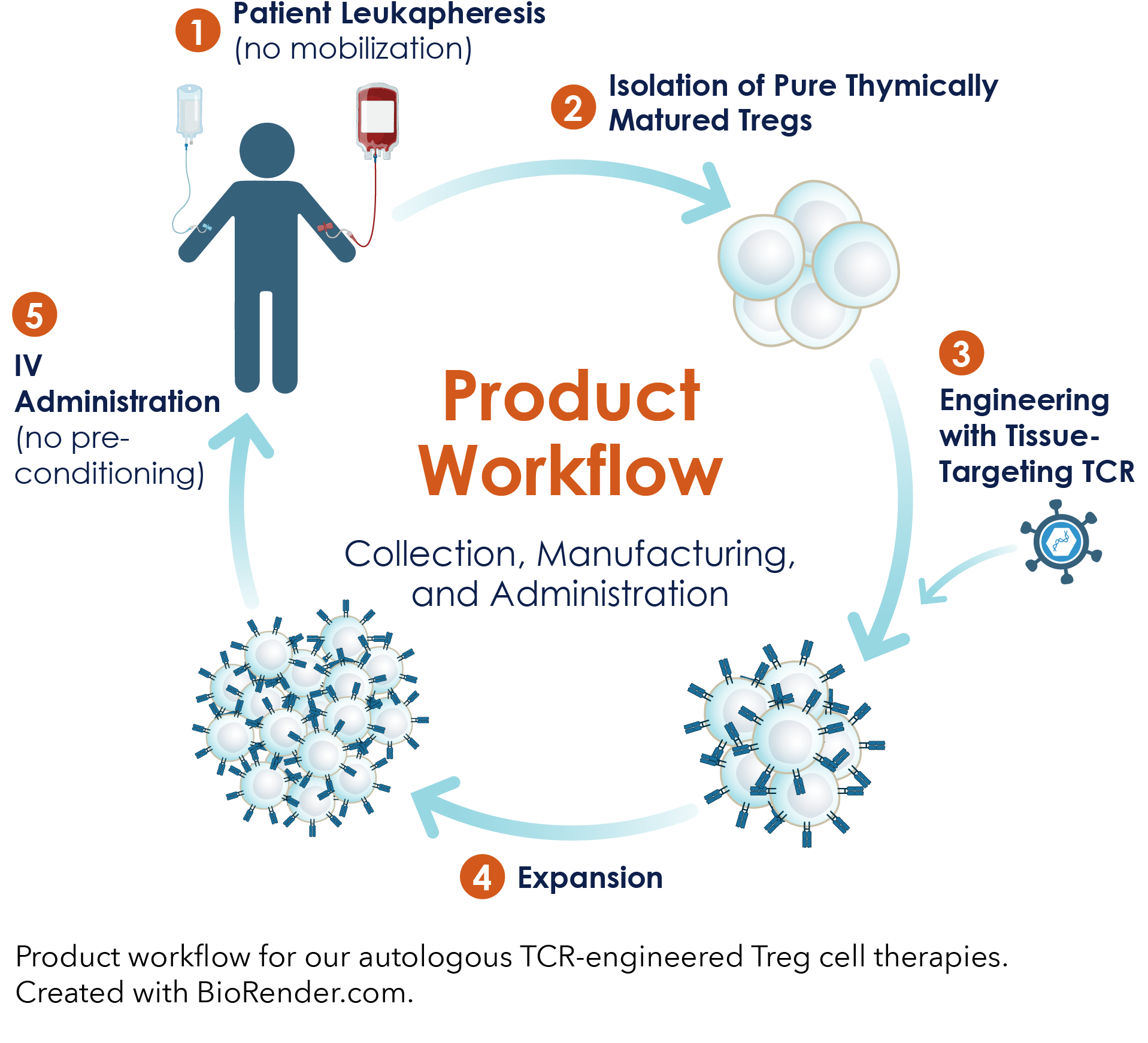
A Robust Cell Therapy Manufacturing Process
A robust, plug-&-play manufacturing process, including bespoke innovations in cell isolation and expansion, to reproducibly create autologous TCR-engineered Treg drug product.
Preclinical Models
Innovative preclinical models that broadly enable the evaluation of TCR-Treg product candidates in any autoimmune indication.
Partnership
![]()
We have partnered with ElevateBio, leveraging ElevateBio BaseCamp to accelerate development of our Treg therapies.
Timeline
The Science Behind Abata’s Approach
Tregs were first identified in the 1990s. Over the next 25 years, the study of Tregs to assess their therapeutic potential expanded greatly. Research has demonstrated they have incredible potential for the treatment of autoimmune diseases, are stable and durable in vivo, and are manufacturable ex vivo. During the same time frame, new insights in multiple sclerosis (MS) and type 1 diabetes (T1D) unveiled the potential for Treg cell therapies to alter the course of these devastating diseases.
The timeline below highlights scientific advances that underlie our therapeutic approach. Additional relevant publications in the scientific literature can be found here.

Mar 2024
Harnessing regulatory T cells to establish immune tolerance
Apr 2023
Opinion & Video: A new MRI technique gives insight into mechanisms underlying MS progression and promises to enhance our ability to perform effective clinical trials.
Mar 2022
Demonstration that meningeal inflammation is associated with chronic-active white matter lesions in MS.
Sep 2021
Reinforcement of the therapeutic strategy to target the adaptive immune system to treat MS.
June 2021
Abata Therapeutics Launches to Usher in New Era of Cell Therapy Using Targeted Regulatory T Cells to Treat Serious Autoimmune and Inflammatory Diseases.
Mar 2020
Demonstration that Tregs can be used to treat autoimmune disease in mice, when engineered to express a disease-specific high-quality TCR.
Dec 2019
Demonstration that chronic-active lesions, which are a manifestation of ongoing tissue injury in progressive MS and can be identified in patients using MRI, correlate with more aggressive disease.
Aug 2019
Demonstration that immunomodulatory antibody teplizumab can delay onset of clinical T1D.
Jan 2019
Review: Targeting autoreactive T cells with Tregs is a valid therapeutic approach in MS due to their role in driving MS inflammation.
Sept 2018
Demonstration that peripheral blood CD4+ T cells of MS patients can be analyzed to identify both the TCRs they express and the antigen(s) they recognize.
Aug 2018
Demonstration of heterologous/infectious tolerance in mouse EAE models of CNS inflammation.
Nov 2015
Demonstration that autologous human Tregs can be vastly expanded ex vivo, safely transfused, and are stable for extended periods in patients.
Sept 2012
Review: T cells circulate through the cerebrospinal fluid in the meningeal compartment to carry out immune surveillance of the brain. This process plays a role in MS pathophysiology.
Feb 2012
First demonstration that stable Tregs originate from the thymus before entering the blood stream and populating other organs.
Sept 2010
Demonstration of Treg stability in vivo in the presence of inflammatory autoimmune conditions.
2007-2019
A series of genetic analyses demonstrated that the inherited susceptibility to MS was conferred by genes that govern immune cell function.
2004
Demonstration that antigen-specific Tregs are much more effective than polyclonal Tregs in preclinical models of autoimmune diabetes.
2003
Demonstration that FoxP3 functions as a key driver of Treg development and phenotype.
Aug 1995
First identification of a suppressive T cell population – renamed regulatory T cells or Tregs.
March 2024
Engineered regulatory T (Treg) cells have emerged as precision therapeutics aimed at inducing immune tolerance while reducing the risks associated with generalized immunosuppression. This Viewpoint highlights the opportunities and challenges for engineered Treg cell therapies in treating autoimmune and other inflammatory diseases.
Science Translational Medicine, 2024: Harnessing regulatory T cells to establish immune tolerance.
Patrick Ho, Ellen Cahir-McFarland – CSO Abata, Jason Fontenot, Tracey Lodie, Adel Nada, Oizhi Tang, Laurence Turka, Jeffrey Bluestone
April 2023
Descriptive categorical classifiers of multiple sclerosis (MS) – relapsing–remitting, chronic-progressive – do not correspond to mechanisms that explain symptomatic worsening. Persons with MS simultaneously exhibit relapse activity with clinical attacks accompanied by new magnetic resonance imaging (MRI) lesions and progression independent of relapse activity (PIRA), each to varying degrees throughout the disease course. Relapse activity has been successfully treated while PIRA has not. PIRA is mechanistically associated with chronic-active lesions (CALs) and cortical demyelination, both causally associated with meningeal lymphoid aggregates, as well as neurite degeneration. Conventional MRI does not detect pathology associated with PIRA except as tissue atrophy but recent advances using MRI to detect CALs may enable a personalized medicine approach to understanding and treating PIRA.
JCI Insight, 2023: Accumulation of meningeal lymphocytes correlates with white matter lesion activity in progressive MS
Richard M. Ransohoff – co-founder of Abata
Watch a conversation with Richard Ransohoff, M.D and John Maraganore, Ph. D. detailing how our new understanding of MS disease pathology and progression can transform therapeutic development.
March 2022
In this study the authors addressed the relationship between meningeal inflammation and demyelinating activity within white matter (WM) lesions, using autopsy-derived brain sections from the indispensable Netherlands Brain Bank. The novelty of this work lay in its focus on the activity of the WM lesions. Previously it was shown that total WM lesion number and volume were unrelated to the extent of meningeal inflammation. Here, Ramaglia et al. showed that WM lesion activity was strongly related to the level of meningeal inflammation – higher meningeal inflammation was associated with actively demyelinating WM lesions, while lower meningeal inflammation was seen in tissues that featured inactive or remyelinating WM lesions.
JCI Insight, 2022: Accumulation of meningeal lymphocytes correlates with white matter lesion activity in progressive MS
Shanzeh Ahmed, Nina Fransen, Hanane Touil, Iliana Michailidou, Inge Huitinga, Jennifer Gommerman, Amit Bar-Or – advisor to Abata , Valeria Ramaglia
September 2021
This study demonstrated that chronic-active lesions in the white matter of multiple sclerosis patients, the neuropathological correlate of paramagnetic rim lesions (PRLs) detected on MRI scans, are driven by the adaptive immune response, and not innate immunity, which has significant implications for drug development strategy.
Nature, September 2021: A lymphocyte–microglia–astrocyte axis in chronic active multiple sclerosis
Martina Absinta, Dragan Maric, Marjan Gharagozloo, Thomas Garton, Matthew Smith, Jing Jin, Kathryn Fitzgerald, Anya Song, Poching Liu, Jing-Ping Lin, Tianxia Wu, Kory Johnson, Dorian McGavern, Dorothy Schafer, Peter Calabresi, Daniel Reich – advisor to Abata
June 2021
Abata Therapeutics Launches to Usher in New Era of Cell Therapy Using Targeted Regulatory T Cells to Treat Serious Autoimmune and Inflammatory Diseases
March 2020
Mouse polyclonal Tregs were isolated, engineered ex vivo with TCRs reacting to one or two myelin-associated antigens, and infused back into mice with autoimmune encephalomyelitis (a model representing the autoimmune features of multiple sclerosis). Whether targeting one or two antigens, in both cases Tregs expressing the high-quality TCRs were most effective in suppressing inflammation and disease score.
Journal of Autoimmunity, March 2020: Treatment of experimental autoimmune encephalomyelitis with engineered bi-specific Foxp3+ regulatory CD4+ T cells
Manish Malviya, Abdelhadi Saoudi, Jan Bauer, Simon Fillatreau, Roland Liblau
December 2019
In MS, chronic-active demyelinating lesions, which previously could only be detected at autopsy, can now be identified on susceptibility-based MRI in vivo as T1-dark foci with paramagnetic rims. Presence and number of these lesions correlate with more severe clinical disease.
JAMA Neurology, December 2019: Association of chronic active multiple sclerosis lesions with disability in vivo
Martina Absinta, Pascal Sati, Federica Masuzzo, Govind Nair, Varun Sethi, Hadar Kolb, Joan Ohayon, Tianxia Wu, Irene Cortese, Daniel Reich – advisor to Abata
August 2019
This clinical study demonstrated that anti-CD3 antibody teplizumab delays onset of clinical T1D in individuals at high risk of developing T1D and formed the basis of its FDA approval in 2022. This finding supports the notion that targeting the underlying autoimmune reponse can modify the clinical disease course of T1D.
New England Journal of Medicine, Aug 2019: An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes
Kevan Herold, Brian Bundy, Alice Long, Jeffrey Bluestone, Linda DiMeglio, Matthew Dufort, Stephen Gitelman, Peter Gottlieb, Jeffrey Krischer, Peter Linsley, Jennifer Marks, Wayne Moore, Antoinette Moran, Henry Rodriguez, William Russell, Desmond Schatz, Jay Skyler, Eva Tsalikian, Diane Wherrett, Anette-Gabriele Ziegler, Carla Greenbaum; Type 1 Diabetes TrialNet Study Group
January 2019
In this article, a pre-eminent neuropathologist who has been investigating MS for more than 40 years reviews data concluding that there are no meaningful biological differences between the autoimmune processes underlying primary progressive multiple sclerosis (PPMS) and secondary progressive MS (SPMS). Furthermore, the reviewer discusses the concept that ‘compartmentalized inflammation’, mainly localized in meningeal lymphoid aggregates (which include T cells), drives ongoing inflammatory demyelination during progressive MS behind an intact blood-brain barrier.
Frontiers in Immunology, Jan 2019: Pathogenic mechanisms associated with different clinical courses of multiple sclerosis
Hans Lassmann
September 2018
In this study, Abata co-founder Roland Martin and colleagues show that memory B cells drive proliferation of self-reactive brain-homing CD4+ T cells, which recognize autoantigens expressed in B cells and in brain lesions with target potential in multiple sclerosis.
Cell, September 2018: Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis
Ivan Jelcic, Faiez Al Nimer, Jian Wang, Verena Lentsch, Raquel Planas, Ilijas Jelcic, Aleksandar Madjovski, Sabrina Ruhrmann, Wolfgang Faigle, Katrin Frauenknecht, Clemencia Pinilla, Radleigh Santos, Christian Hammer, Yaneth Ortiz, Lennart Opitz, Hans Gronlund, Gerhard Rogler, Onur Boyman, Richard Reynolds, Andreas Lutterotti, Mohsen Khademi, Tomas Olsson, Fredrik Piehl, Mireia Sospedra, Roland Martin – co-founder of Abata
August 2018
This study demonstrates that T cells do not need to see the disease-driving antigen to confer efficacy in mouse EAE models. The authors utilize an MBP-specific TCR and observed that it can suppress MOG-induced/driven EAE in the mouse, thus demonstrating heterologous/infectious tolerance. The work teaches that T cells do not need to ‘see’ the disease-driving antigen: hence, a TCR can direct the Treg to the inflamed brain lesion and become locally reactivated to suppress immunity even if aimed at other antigens in the same microenvironment.
Journal of Autoimmunity, August 2018: Engineered MBP-specific human Tregs ameliorate MOG-induced EAE through IL-2-triggered inhibition of effector T cells
Yong Chan Kim, Ai-Hong Zhang, Jeongheon Yoon, William Culp, Jason Lees, Kai Wucherpfennig, David Scott
November 2015
A clinical study in Type 1 diabetes patients shows autologous Tregs can be vastly expanded ex vivo and safely transfused, retaining high numbers and a stable Treg phenotype for at least one year.
Science Translational Medicine, November 2015: Type 1 diabetes immunotherapy using polyclonal regulatory T cells
Jeffrey Bluestone, Jane Buckner, Mark Fitch, Stephen Gitelman, Shipra Gupta, Marc Hellerstein, Kevan Herold, Angela Lares, Michael Lee, Kelvin Li, Weihong Liu, Alice Long, Lisa Masiello, Vinh Nguyen, Amy Putnam, Mary Rieck, Peter Sayre, Qizhi Tang
September 2012
Richard Ransohoff, co-founder of Abata, and Britta Engelhardt reviewed data leading to the concept that memory T cells traffic through the meningeal compartment to execute immune surveillance of the central nervous system (CNS). These T cells, previously stimulated by cognate antigen in the periphery, are restimulated within the CNS, primarily by antigen-MHC complexes on the surfaces of meningeal macrophages, which sample solutes in the cerebrospinal fluid (CSF). This paradigm provides understanding of how peripherally administered TCR-expressing Tregs can traffic through the compartment and be activated by antigen and ultimately retained locally.
Nature Reviews: Immunology, Sept. 2012: The anatomical and cellular basis of immune surveillance in the central nervous system
Richard M. Ransohoff – co-founder of Abata, Britta Engelhardt
February 2012
Treg cells with a stable, terminally differentiated immunosuppressive phenotype (‘stable Tregs’) originate in the thymus before entering the blood stream and populating other organs in the body. Although Tregs express the key transcription factor FoxP3, it is also shown that under specific conditions FoxP3 is temporarily induced in effector T cells, without taking on a regulatory phenotype.
Immunity, February 2012: Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells
Takahisa Miyao, Stefan Floess, Ruka Setoguchi, Herve ́ Luche, Hans Joerg Fehling, Herman Waldmann, Jochen Huehn, Shohei Hori
September 2010
Studying the cellular origin and fate of Treg cells in mice, Abata co-founder Diane Mathis and collaborating scientists definitively demonstrate that a FoxP3-expressing Treg lineage can be identified. Born as a regulatory cell, it retains a stable regulatory cell phenotype and will not convert to an effector T cell phenotype even when exposed to highly pro-inflammatory conditions.
Science, September 2010: Stability of the regulatory T cell lineage in vivo
Yuri Rubtsov, Rachel Niec, Steven Josefowicz, Li Li, Jaime Darce, Diane Mathis – co-founder of Abata, Christophe Benoist, Alexander Rudensky
2007-2019
Decades of research using animal models including EAE had suggested strongly that T cell-mediated autoimmunity was the underlying pathogenic process in MS (see the 1981 reference below for an important early study).1 These human genetic studies confirmed that hypothesis by showing the inherited susceptibility to MS was conferred by genes that govern the function of immune cells, most prominently T cells. Additional research showed both peripheral immune cells and brain immune cells termed microglia were also involved.
New England Journal of Medicine, 2007: Risk alleles for multiple sclerosis identified by a genomewide study
International Multiple Sclerosis Genetics Consortium, David Hafler, Alastair Compston, Stephen Sawcer, Eric Lander, Mark Daly, Philip De Jager, Paul de Bakker, Stacey Gabriel, Daniel Mirel, Adrian Ivinson, Margaret Pericak-Vance, Simon Gregory, John Rioux, Jacob McCauley, Jonathan Haines, Lisa Barcellos, Bruce Cree, Jorge Oksenberg, Stephen Hauser
Nature, 2011: Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis
The International Multiple Sclerosis Genetics Consortium, The Wellcome Trust Case Control Consortium
Science, 2019: Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility
The International Multiple Sclerosis Genetics Consortium
1Journal of Immunology, October 1981: Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2- T lymphocytes
Carla Pettinelli, Dale McFarlin
2004
Tregs are shown to potently suppress autoimmune diabetes in mice following adoptive transfer, revealing a potential novel avenue for immunotherapy of human autoimmune disease. Successful in vitro expansion of antigen-specific Tregs yielded significant efficacy, reversing diabetes even after onset.
Journal of Experimental Medicine, 2004: CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes
Kristin Tarbell, Sayuri Yamazaki, Kara Olson, Priscilla Toy, Ralph Steinman
Journal of Experimental Medicine, 2004: In vitro–expanded antigen-specific regulatory T cells suppress autoimmune diabetes
Qizhi Tang, Kammi Henriksen, Mingying Bi, Erik Finger, Gret Szot, Jianqin Ye, Emma Masteller, Hugh McDevitt, Mark Bonyhadi, Jeffrey Bluestone
2003
These two papers show the transcription factor Foxp3 is specifically expressed in Tregs, acting as a critical regulator of their development and function. Mice and human subjects carrying genetic defects in Foxp3 are demonstrated to develop autoimmune and inflammatory syndromes.
Nature Immunology, 2003: Foxp3 programs the development and function of CD4+CD25+ regulatory T cells
Jason Fontenot, Marc Gavin, Alexander Rudensky
Science, 2003: Control of regulatory T cell development by the transcription factor Foxp3
Shohei Hori, Takashi Nomura, Shimon Sakaguchi
August 1995
First convincing demonstration of immune suppressive capacity encased in a CD4+ T cell population identified by constitutive expression of CD25. Removing this subset of suppressive T cells led to a wide variety of autoimmune diseases in mouse experiments. This subset of CD4+ T cells was later named “regulatory T cells” or “Tregs”.
Journal of Immunology, August 1995: Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases.
Shimon Sakaguchi, Noriko Sakaguchi, Masanao Asano, Misako Itoh, Masaaki Toda